Abstract

Background: Glofitamab, a CD20xCD3 T-cell-engaging bispecific monoclonal antibody with a novel 2:1 (CD20:CD3) configuration, redirects T cells to eliminate normal and malignant B cells. Glofitamab is an off-the-shelf treatment, administered intravenously (IV) for a fixed duration of 12 cycles. In pivotal expansion cohorts of an ongoing Phase I/II study (NP30179; NCT03075696), glofitamab has demonstrated a manageable safety profile and induced frequent and durable complete responses (CRs) in patients with relapsed/refractory (R/R) large B-cell lymphoma (LBCL; Dickinson et al. ASCO 2022). Here, we present data for the duration of CR from end-of-treatment (EOT) in patients with R/R LBCL enrolled in the dose escalation and expansion phases of this study.
Methods: Patients with R/R LBCL with ≥1 prior line of therapy (diffuse large B-cell lymphoma not otherwise specified [DLBCL NOS], high-grade B-cell lymphoma [HGBCL], primary mediastinal large B-cell lymphoma [PMBCL] or transformed follicular lymphoma [trFL]) were enrolled. Patients received 1000mg obinutuzumab pre-treatment 7 days prior to first dose of glofitamab, followed by IV glofitamab for up to 12 cycles (8.4 months). Glofitamab was given at a fixed dose (0.6-25mg) or with step-up dosing (target dose: 16mg or 30mg) every three weeks. Responses were assessed using Lugano 2014 criteria (Cheson et al. J Clin Oncol 2014).
Results: As of March 14, 2022, 61/214 (29%) patients with R/R LBCL who had received glofitamab at a dose of ≥0.6mg, including suboptimal doses, were in CR by investigator assessment at EOT by intent-to-treat analysis (DLBCL, n=41; trFL, n=18; HGBCL, n=1; PMBCL, n=1). Median age was 67 years (range: 22-85), and 51% were female. The median number of prior lines of therapy was 3 (range: 2-9), and the majority of patients (61%) had received ≥3 prior therapies. Overall, 43% of patients were refractory to their initial therapy, and 74% were refractory to their most recent regimen.
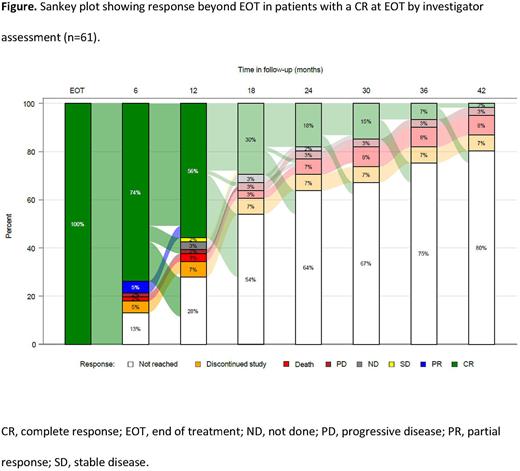
The median duration of CR follow-up was 18.1 months (95% confidence interval [CI]: 14.8-20.7). Fifty-three patients had reached 6 months follow-up post-EOT; the majority of patients with a CR at EOT (45/61; 74%) remained in CR, 1/61 (2%) had experienced progressive disease (PD), and 8/61 (13%) remained in follow-up but had not yet reached 6 months. At 12 months post-EOT, 34/61 (56%) patients remained in CR, 1/61 (2%) had experienced PD, and 17/61 (28%) remained in follow-up but had not yet reached 12 months. One patient experienced PD between 12 and 18 months post-EOT (Figure). Forty-six percent of patients had been in follow-up long enough to reach their 18-month post-EOT visit, and 20% of patients had reached their 42-month post-EOT visit. The median duration of CR had not been reached. Of the three patients who experienced PD after having a CR at EOT, one patient had initiated re-treatment at the time of the analysis and achieved a second CR. Five patients discontinued the study (two received an allogeneic transplant, two due to physician decision, one lost to follow-up). Five patients died (one due to secondary malignancy, two due to PD, two for unknown/other reasons).
Conclusions: Most patients with LBCL who achieved a CR at EOT experienced durable responses in the absence of continued treatment. Only 1 of 44 patients who reached 12 months follow-up post-EOT experienced progression. Longer follow-up is needed to further confirm the off-treatment durability of glofitamab-induced CRs in patients with R/R LBCL beyond the 12-month timepoint. It is particularly encouraging that off-treatment progression was rarely observed in this heavily pre-treated, largely treatment-refractory, LBCL patient population.
Disclosures
Hutchings:Celgene: Consultancy, Research Funding; Novartis: Research Funding; Genmab: Consultancy, Research Funding; Genentech: Research Funding; Janssen: Consultancy, Research Funding; Incyte: Research Funding; Roche: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; AbbVie, Celgene, Genmab, Janssen, Roche, Takeda: Membership on an entity's Board of Directors or advisory committees; Celgene, Genentech, Genmab, Incyte, Janssen, Novartis, Roche, Takeda: Research Funding; AbbVie: Consultancy. Carlo-Stella:Bristol Myers Squibb: Honoraria; ADC Therapeutics: Honoraria, Other: Consultancy/Advisory, Research Funding; Sanofi: Other: Consultancy/Advisory, Research Funding; Merck Sharp & Dohme: Honoraria; Janssen Oncology: Honoraria; Roche: Other: Consultancy/Advisory, Research Funding; Celgene/Bristol Myers Squibb: Other: Consultancy/Advisory; Takeda: Honoraria; Novartis: Honoraria; Incyte: Honoraria; AstraZeneca: Honoraria; Karyopharm Therapeutics: Other: Consultancy/Advisory; Scenic Biotech: Other: Consultancy/Advisory. Morschhauser:AstraZeneca: Membership on an entity's Board of Directors or advisory committees; Allogene therapeutics: Membership on an entity's Board of Directors or advisory committees; Miltenyi: Membership on an entity's Board of Directors or advisory committees; Janssen: Speakers Bureau; Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Epizyme: Consultancy, Membership on an entity's Board of Directors or advisory committees; Genmab: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Genentech: Consultancy; Bristol Myers Squibb: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Gilead Sciences: Consultancy, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees. Bachy:Kite, Gilead, Novartis, Roche, Incyte, Miltenyi Biotech, Takeda, Sanofi: Honoraria; Roche, Gilead, ADC Therapeutics, Takeda, Novartis, Incyte: Membership on an entity's Board of Directors or advisory committees; Amgen, BMS: Research Funding; Hospices Civils de Lyon: Current Employment. Corradini:takeda: Honoraria; janssen: Honoraria; incyte: Honoraria; gilead: Honoraria; celgene: Honoraria; amgen: Honoraria; abbvie: Honoraria. Iacoboni:NOVARTIS, KITE/GILEAD, BMS/CELGENE: Consultancy; NOVARTIS, KITE/GILEAD, BMS/CELGENE, ASTRAZENECA, ROCHE, ABBVIE, JANSSEN, MILTENYI: Honoraria. Khan:Allegheny Health Network, Pittsburgh, PA: Current Employment; Acceleron, AstraZeneca, Beigene, Sanofi: Consultancy; Genentech, Beigene: Research Funding; BMS, AstraZeneca, Beigene, Sanofi, Incyte, Amgen, Genentech, Epizyme, Karyopharm, SeaGen, Pharmacyclics, Janssen: Honoraria, Speakers Bureau. Patel:AstraZenca, BMS, Celgene, Roche/Gen, Kite, Pharmacyclics/Janssen, TG: Speakers Bureau; Adaptive Biotechnologies, AptevoTherapeutics, AstraZeneca, BMS, Celgene, CRISPR, Curis, Epizyme, Fate Therapeutics, Roche/Gen, Kite, MEI, Nurix, Pharmacyclics/Janssen, Sunesis Pharmaceuticals, Trillium Therapeutics/Pfizer, Velos Bio, Xencor: Research Funding; Abbvie, ADC Therapeutics, AstraZeneca, Beigene, BMS, Caribou Biosciences, Celgene, Epizyme, Roche/Gen, Kite, MEI, Morphosys, Pharmacyclics/Janssen, TG, Trillium Therapeutics/Pfizer, Xencor: Consultancy. Hertzberg:Takeda: Membership on an entity's Board of Directors or advisory committees; Otsuka: Membership on an entity's Board of Directors or advisory committees; Beigene: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Mundipharma: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees; Roche, Takeda, Otsuka, Beigene, Gilead, Janssen, Novartis, Mundipharma: Honoraria, Membership on an entity's Board of Directors or advisory committees. Falchi:ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees; Genetech: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; Roche: Consultancy, Research Funding; Genmab: Consultancy, Research Funding. Bartlett:Autolus, Bristol-Meyers Squibb, Celgene, Forty Seven, Janssen, Kite Pharma, Merck, Millennium, Pharmacyclics: Research Funding; Washington University School of Medicine: Current Employment; ADC Therapeutics, Roche/Genentech, Seattle Genetics: Membership on an entity's Board of Directors or advisory committees, Research Funding. Brody:Genentech: Research Funding; Gilead/Kite: Research Funding; Merck: Research Funding; SeaGen, Roche, Genentech, Merck, ADC Therapeutics, Epizyme, Gilead, Kite, Astrazeneca: Research Funding; BMS: Research Funding; Seagen: Consultancy; ADC Therapeutics: Consultancy; Epizyme: Consultancy. Lundberg:F-Hoffmann La Roche: Current Employment, Current equity holder in private company, Current equity holder in publicly-traded company, Current holder of stock options in a privately-held company, Divested equity in a private or publicly-traded company in the past 24 months, Patents & Royalties. Xie:Roche: Current Employment. Mulvihill:Hoffmann-La Roche: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company, Divested equity in a private or publicly-traded company in the past 24 months, Ended employment in the past 24 months. Baumlin:Hoffmann La Roche: Current Employment. Relf:Roche, F-Star Therapeutics: Current equity holder in publicly-traded company; Roche, F-Star Therapeutics and Harpoon Therapeutics: Divested equity in a private or publicly-traded company in the past 24 months; Roche Products Limited: Current Employment. Piccione:F. Hoffman-LaRoche Ltd: Current equity holder in publicly-traded company; Genentech, Inc: Current Employment. Humphrey:Roche Products Ltd: Current Employment; Roche: Current equity holder in private company, Current holder of stock options in a privately-held company. Dickinson:Roche, BMS, Novartis, Kite, Gilead, NKARTA, AdiCet Bio, Interius, Janssen, MSD: Consultancy; Roche, BMS, Novartis, Kite, Gilead, NKARTA, AdiCet Bio, Interius, Janssen, MSD, Amgen: Honoraria; Roche, Novartis, Kite, Gilead, MSD, Takeda, Celgene: Research Funding.
OffLabel Disclosure:
Glofitamab is a full-length, humanized immunoglobulin G1 bispecific monoclonal antibody with a 2:1 (CD20:CD3) configuration that facilitates bivalent binding to CD20 on B cells, and monovalent binding to CD3 on T cells. Glofitamab redirects T cells to eliminate normal and malignant B cells. Glofitamab is an investigational agent. Obinutuzumab (Gazyva) is a CD20-directed cytolytic antibody indicated: in combination with chlorambucil, for the treatment of patients (pts) with previously untreated CLL; in combination with bendamustine followed by obinutuzumab monotherapy, for the treatment of pts with FL who relapsed after, or are refractory to, a rituximab-containing regimen; in combination with chemo followed by obinutuzumab monotherapy in pts achieving at least a PR, for the treatment of adult pts with previously untreated stage II bulky, III or IV FL.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal